Chugai and NTT DATA Complete Demonstration of AI-based Clinical Trial Efficiency Solution
September 9, 2020
Chugai Pharmaceutical Co., Ltd.
NTT DATA Corporation
TOKYO, September 9, 2020 - Chugai Pharmaceutical Co., Ltd. (TOKYO: 4519) and NTT DATA Corporation (TOKYO: 9613) have conducted a demonstration test of a solution for improving the efficiency of clinical trial-related document preparation using AI technology from January to June, 2020.
Clinical trial operations require the creation of a variety of documents and procedures from planning to implementation and application for drug approval. NTT DATA has been developing a solution based on the concept of improving the process efficiency of documentation, for example, by automatically creating clinical trial-related documents from a clinical trial protocol1). In this demonstration, Chugai and NTT DATA are collaborating by conducting concept verification, evaluating the usefulness of this solution and identifying future system issues.
Based on the results of this demonstration, NTT DATA aims to provide commercial services of this solution in FY2021. Furthermore, based on the cooperative relationship between the two companies obtained through this demonstration, we will collaborate and accelerate our efforts toward digital transformation of clinical development operations.
Background
New drug development is a process that involves research, collection and analysis of clinical data in clinical trials, and evaluation of efficacy and safety. It usually takes about 9 to 17 years on average to launch a new drug2). Both pharmaceutical companies and medical institutions participating in clinical studies have expended so much effort to ensure the quality of clinical trial activities and data. In addition, efficient development of high-quality clinical trial-related documents is a common challenge among pharmaceutical companies.
NTT DATA is developing a cross-pharmaceutical company/clinical site total solution platform for clinical trials to connect a wide range of clinical trial processes through the most advanced IT technology to help accelerate new drug development.
Under the goal of optimization of all value chains, one of the basic strategies of CHUGAI DIGITAL VISION 20303), Chugai aims at further acceleration of clinical trial processes. Chugai supports NTT DATA's initiative and conducted the demonstration test jointly with NTT DATA to develop a new solution using AI technology, leveraging Chugai's extensive experience.
About the solution for increasing the Efficiency of Clinical Trial-Related Document Development
The solution, as one of total solution platforms for clinical trials, allow sequential and comprehensive generation of various clinical trial-related documents (such as informed consent form for patients, statistical analysis plan, case report form to enter patients' clinical trial data, and clinical study report summarizing the clinical trial results), using data from the protocol as input information, through AI technology, as well as technology components such as ontology4) and semantics5).
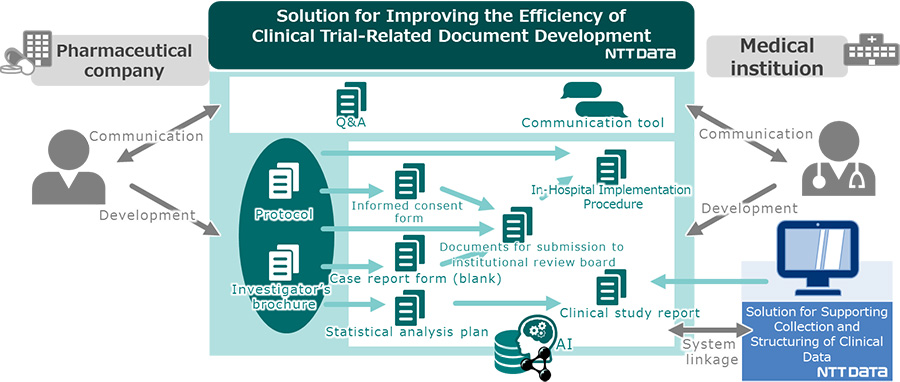
Figure 1 Overview of the solution for improving the efficiency of clinical trial-related document development
About the demonstration test
| Purpose | To identify requirements for solving the challenges in efficient development of high-quality clinical trial-related document, aiming at digital transformation in clinical development activities. |
| Procedure | To validate the usefulness of the concept to sequentially and automatically generate clinical trial-related documents required for clinical development activities, and to evaluate technology components for realization. Specifically, we compared time required for the preparation of the informed consent form and the case report form (blank) from Chugai's protocol as input information, using the prototype of the solution, with the actual time spent for the comparable activities in the past. |
| Period | From January to June 2020 |
| Validation Results | The usefulness of this solution's concept was successfully validated identifying tasks ahead. The validation results of the effectiveness are as follows;
|
About Future Development
Leveraging the technology components validated in the demonstration test, NTT DATA will expand the applicable clinical trial-related documents, provide a standardized template, and implement suggest functions with AI6) and multiple language support functions sequentially in the solution. In addition to the solution, NTT DATA will seek to provide a total solution platform for clinical trials, including the solution for supporting collection and structuring of clinical data. Chugai and NTT DATA are working together toward the digital transformation of clinical development operations and contributing to the creation of a new framework for more efficient drug development.
Comments from Both Companies
Efficient development of high-quality clinical trial-related documents is a common challenge among pharmaceutical companies. We are very pleased that Chugai's expertise and experience accumulated in clinical development may contribute to the initiative to accelerate new drug development by building a shared clinical trial platform for pharmaceutical companies and medical institutions with the use of AI technology. Under CHUGAI DIGITAL VISION 2030, Chugai has set its goal to be a top innovator that will provide society-changing healthcare solutions by transforming our business. We will continue to actively work with external partners to lead innovation in the healthcare industry.
Satoko Shisai, Chugai's Vice President, Head of Digital & IT Supervisory Division
We sincerely appreciate that we have successfully completed the demonstration test with Chugai aiming toward digital transformation in clinical development activities. We hope that our innovative solutions developed through our collaboration will resolve the common challenge in clinical development activities in the pharmaceutical industry and help build a foundation to ensure the swift delivery of new drugs to patients. NTT DATA is committed to realizing a healthy longevity society and secure and safe living, as well as contributing to the creation of the future society with customers, using advanced IT technology.
Hidenori Chihara, NTT DATA's Executive Vice President, Head of Public Sector 2
Note:
1)Protocol is a document described requirements for the clinical trial, for example, the purpose and procedures of the clinical trial
2)Source: Japan Pharmaceutical Manufacturers Association (JPMA). http://www.jpma.or.jp/medicine/med_qa/info_qa55/q33.html, (Japanese only) (Reference September 1, 2020)
3)About CHUGAI DIGITAL VISION 2030
In CHUGAI DIGITAL VISION 2030, Chugai has set its goal for 2030 to transform its business by using digital technologies to make Chugai a top innovator in the provision of society-changing healthcare solutions. Based on the three basic strategies consisting of 1) Strengthen digital platform, 2) Optimize all value chains, and 3) Digital transformation for drug discovery and development, the company aims to transform its business and provide society-changing healthcare solutions.

- CHUGAI DIGITAL from Official Website of Chugai (https://www.chugai-pharm.co.jp/english/profile/digital/index.html)
- The Future of Healthcare Transformed by Digital Technologies | CHUGAI DIGITAL Concept Movie (https://youtu.be/SWOYtFX5-1U) (Japanese only)
4)An ontology is data modeling of conceptualization of human knowledge, in other words, a technology which allows us to define relationships and expand knowledge.
5)Semantics is a data structure constructed by connecting meta data (subject, predicate, and object) with data to make it meaningful, suitable to express the relationship between things.
6)Suggest functions are functions to display words and phrases closely related to the character string searched using sequential prediction.
* Every company name, and corporate name described above is a trademark or registered trademark of either company.
Contact Information on the Press Release
Chugai Pharmaceutical Co., Ltd.
Contact from the press
Media Relations Group, Corporate Communications Dept.
Tel: +81-3-3273-0881
E-mail: [email protected]
NTT DATA Corporation
Public Welfare IT Services Division, Public Sector 2
Contact: [email protected]
About Chugai
Chugai Pharmaceutical is one of Japan's leading research-based pharmaceutical companies with strengths in biotechnology products. Chugai, based in Tokyo, specializes in prescription pharmaceuticals and is listed on the 1st section of the Tokyo Stock Exchange. As an important member of the Roche Group, Chugai is actively involved in R&D activities in Japan and abroad. Specifically, Chugai is working to develop innovative products which will satisfy unmet medical needs.
URL: https://www.chugai-pharm.co.jp/english/
About NTT DATA
NTT DATA – a part of NTT Group – is a trusted global innovator of IT and business services headquartered in Tokyo.
We help clients transform through consulting, industry solutions, business process services, IT modernization and managed services. NTT DATA enables clients, as well as society, to move confidently into the digital future. We are committed to our clients' long-term success and combine global reach with local client attention to serve them in over 50 countries.
Visit us at nttdata.com.
News Releases.
The services, prices of products and services, specifications, telephone numbers, etc. for inquiries and other information included in news releases are the data available on the day of the release. This information may be changed at any time without notice. In certain circumstances, due to various risks or unexpected occurrences, actual results may also be different from the plans or projections in news releases.